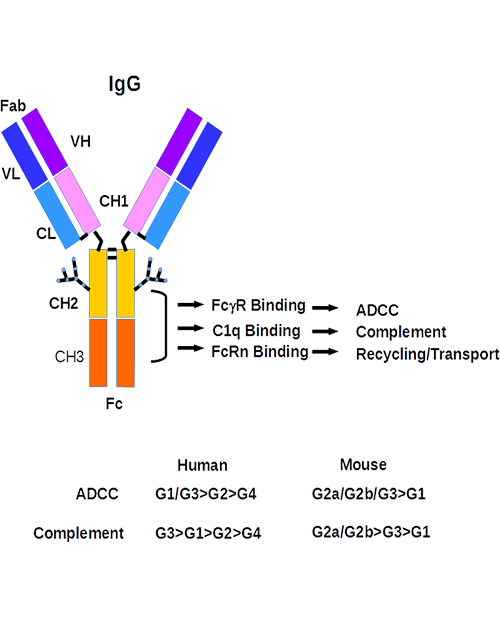
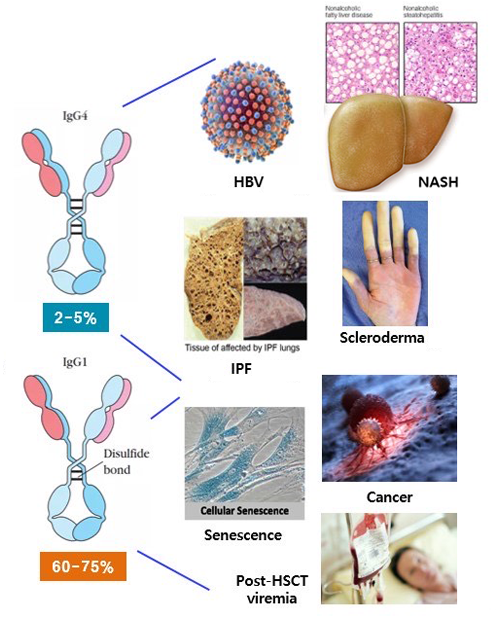
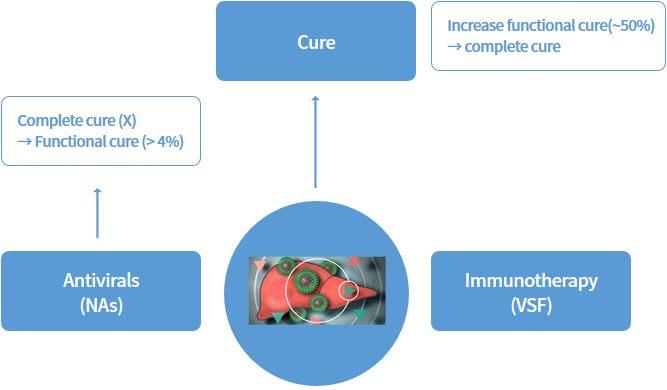
Antivirals
Various synthetic drugs and human-derived interferon are currently used as antiviral drugs, but they have many limitations in clinical practice. Synthetic drugs have a problem of increasing tolerability when used continuously, and interferon is used for various types of viruses, but its effectiveness is quite limited, and several side effects have been reported. We have discovered VSF (Virus Suppressing Factor) that can overcome the shortcomings of existing antiviral agents, and we are developing it as an antiviral agent.